1. Organic Components
Functional Organic Group (Y-group):
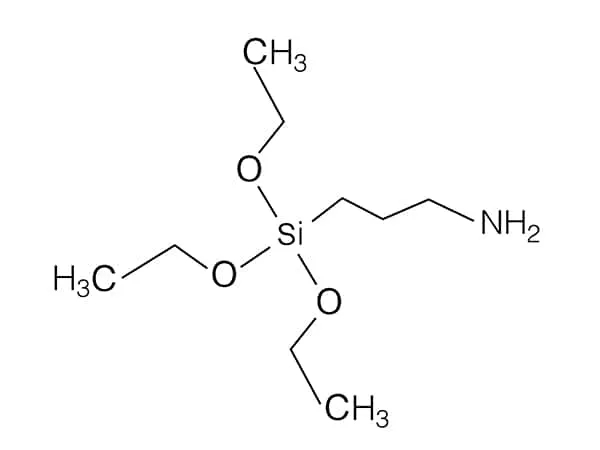
The "Y" in the general formula Y─R─Si(OR')₃ is an organofunctional group that determines reactivity with organic matrices. Common examples include:
Amino (-NH₂): Bonds with epoxy resins or polyamides.
Epoxy: Reacts with polyester or acrylic polymers.
Vinyl (-CH=CH₂): Participates in free-radical polymerization.
Methacrylate: Compatible with UV-curable coatings.
Alkyl Spacer (R-group):
A short hydrocarbon chain (e.g., -CH₂CH₂-) that links the Y-group to the silicon atom, providing flexibility and steric control.
2. Inorganic Components
Silicon-Based Head (Si(OR')₃):
The inorganic portion consists of a silicon atom bonded to three hydrolyzable alkoxy groups (e.g., -OCH₃, -OC₂H₅). These groups undergo hydrolysis to form reactive silanol (-Si-OH) groups, which bond to inorganic surfaces via Si-O-M covalent bonds (where M = metal, glass, or mineral).
3. Hybrid Behavior in Action
Inorganic Bonding:
Hydrolyzed silanol groups form covalent bonds with hydroxylated inorganic surfaces (e.g., glass, metals, silica).
Organic Bonding:
The Y-group reacts with organic materials (polymers, adhesives) through chemical reactions (e.g., condensation, copolymerization).
4. Why This Hybrid Nature Matters
Interfacial Reinforcement:
Silane coupling agents strengthen composites (e.g., fiberglass-reinforced plastics) by chemically linking dissimilar materials.
Moisture Resistance:
The inorganic Si-O-M bonds resist hydrolysis, enhancing durability in humid environments.
Adhesion Promotion:
Used in coatings, adhesives, and sealants to improve bonding to metals, glass, and ceramics.